What’s the Correct Power Supply for a Medical Application?
When it comes to medical applications, engineering teams quickly find themselves sinking into a sea of standards and regulations. There are good reasons for this. Patients may be attached to a medical device long-term, unconscious, and unable to react to an equipment failure. Because of the risks and necessary approvals, it makes sense to get support if your team is tackling such an application for the first time. And, when it comes to power supply selection, this blog post should get you on the right path.
Medical Standards and Terms: an Alphabet-Soup
If you’ve already suspected that there is no such thing as a universal “medical power supply,” you’d be correct. Your team’s approach to PSU selection will be guided by your electrical, mechanical, and budgetary constraints and the specifics of your application’s use case. Central to the decision-making process is IEC 60601. Part 1 covers general requirements, from EMC and radiation to usability. Part 2 focuses on particular requirements for specific products, such as ventilators, infusion pumps, and defibrillators. Despite being in existence for more than 40 years, the standard has kept with the times, reflecting how and where today’s medical equipment is used and deployed.
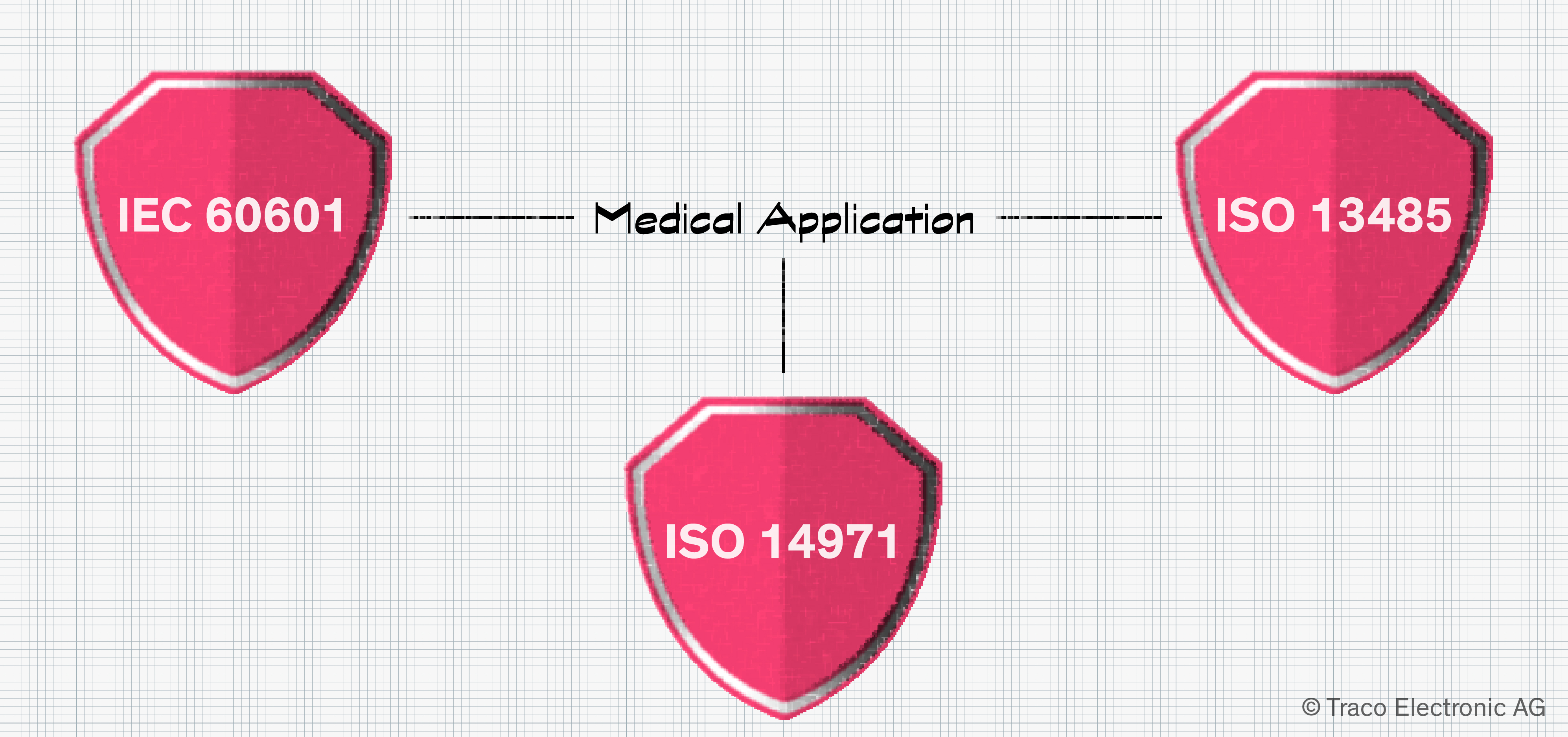
Formal risk assessment is covered in ISO 14971. Minimizing risk is an important element in managing medical products over their life cycle, and this standard defines best practices. Finally, it needs to be shown that a Quality Management System (QMS) is in place that complies with ISO 13485. This doesn’t just apply to the device manufacturer but to vendors of the power supplies selected too.
What are Applied Parts?
The next question to answer is whether the medical electrical equipment or system, during normal use, will come into contact with the patient or an operator. Within IEC 60601, these are defined as applied parts, shortened to AP, and are an essential definition when discussing the risks and defining requirements.
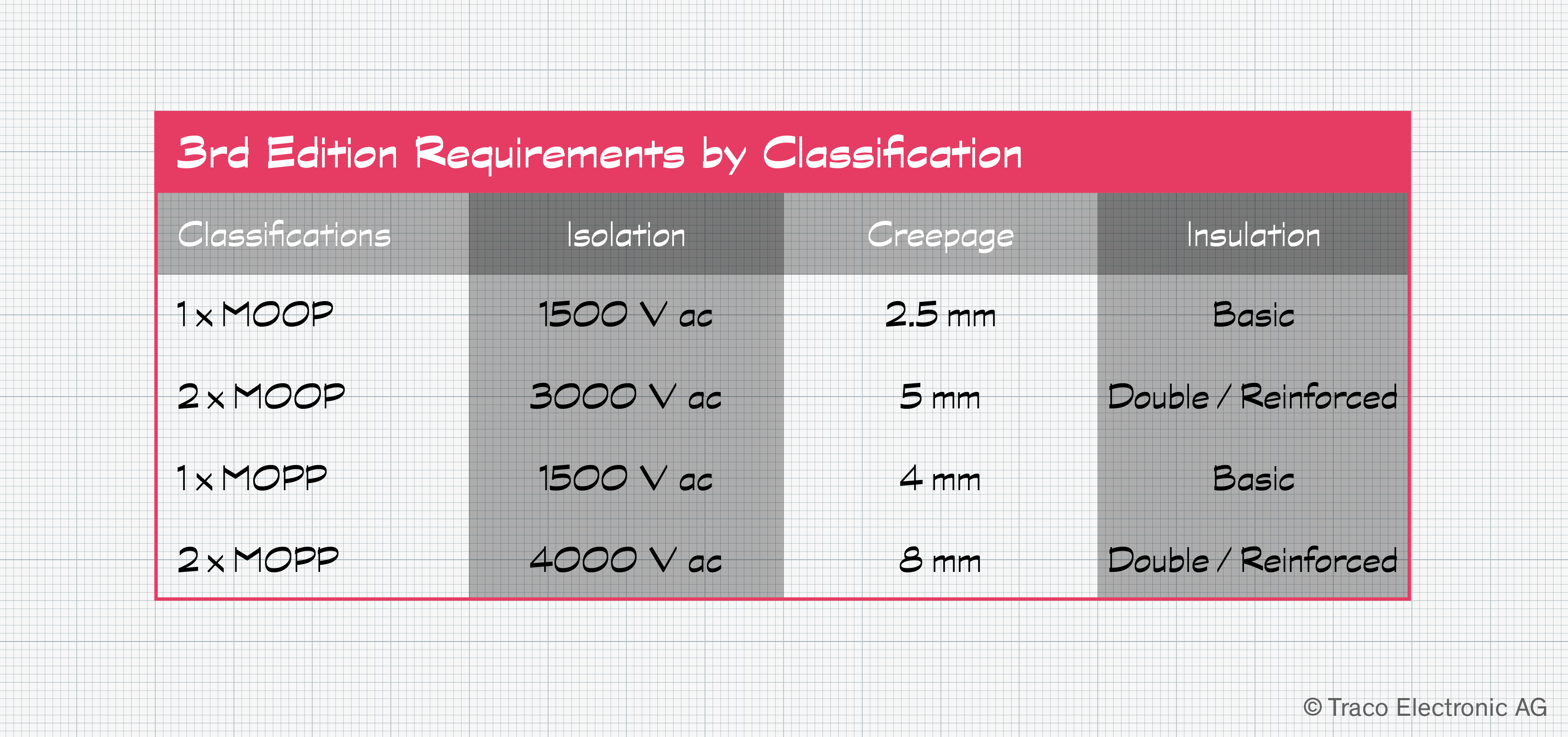
A Means of Protection, or MOP, is required to mitigate such risks. Such protection affords a safeguard against electrical shocks, even under fault conditions. Some procedures, such as cauterization and defibrillation, rely on high voltages. These could be injected into attached medical equipment via the patient, so this path must also be considered to protect the operator. MOP is achieved using methods such as creepage distances, air gaps, safety insulation, and a protective earth, either in isolation or in combination.
The precise requirements depend on who is the focus of the protection. Patients are covered by Means of Patient Protection, or MOPP, whereas operators are covered by Means of Operator Protection, or MOOP. Since patients connected to an AP may be unconscious or have limited mobility, MOPP requirements are the more stringent of the two.
Not all APs are the same, being classified into three different types:
-
Type B – Earth referenced without contact to the patient.
-
Type BF – Connected electrically to the patient but not directly to the heart.
-
Type CF – Connected electrically to the patient’s heart.
IEC 60601 defines maximum earth, enclosure, and patient leakage currents for each type for normal and single fault conditions. The same applies to isolation levels, with BF/CF rated devices having more stringent requirements than B rated applications.
Is a Power Supply an Applied Part?
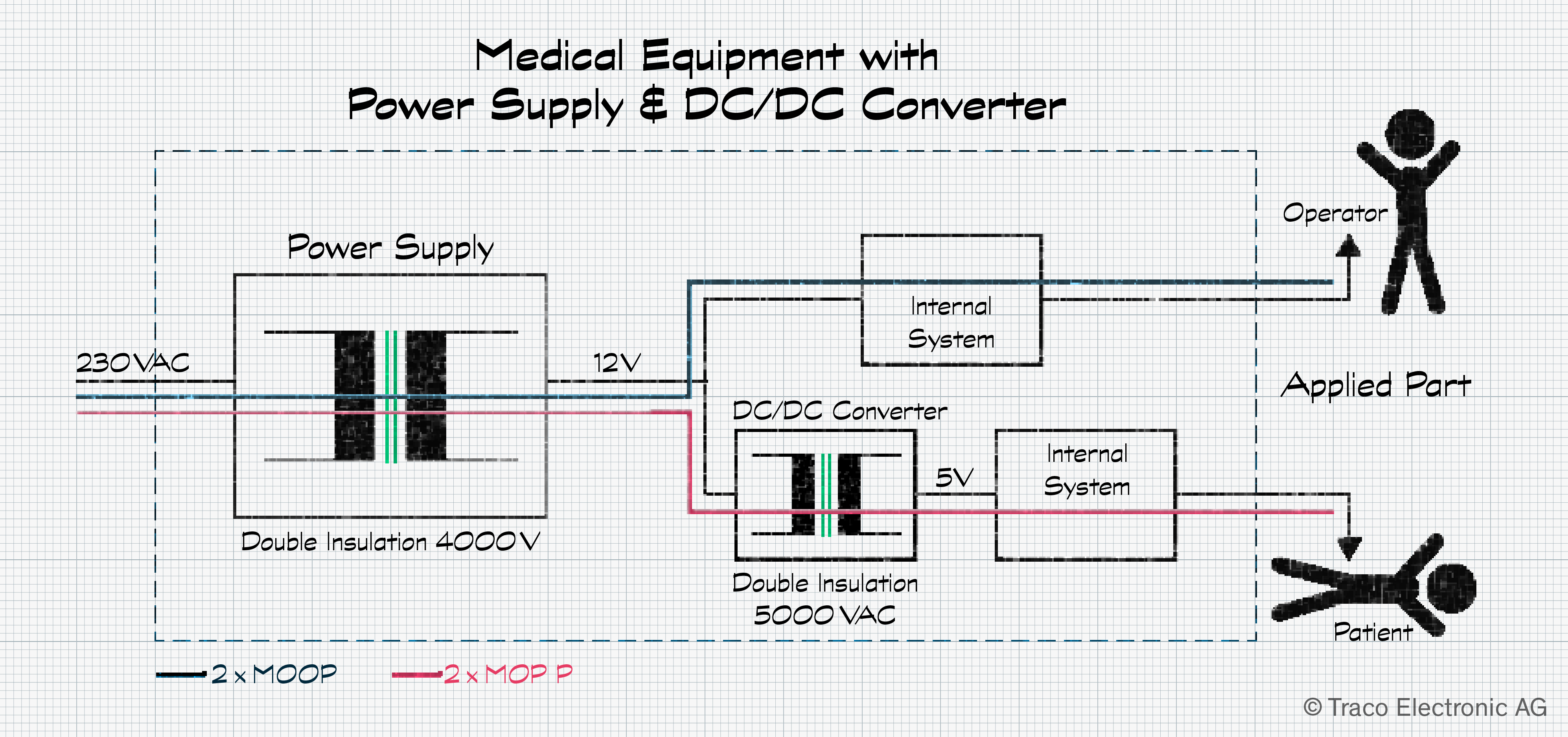
While some power supply models are designed for medical applications, only the part that comes into contact with the patient or operator is considered an applied part. As a result, attaining 2 × MOPP can be implemented in two stages rather than with a single AC/DC supply. The first stage uses a suitable AC/DC power supply with double insulation that fulfills the 2 × MOOP for the operator. The second stage deploys a DC/DC converter meeting the 2 × MOPP needs of the patient path.
This means that AC/DC power supplies meeting IEC 62368-1, a standard pertaining to information technology equipment, can be used if these meet the system’s other power requirements.
How Does a Power Supply Vendor Help Medical Application Developers?
As has become clear, there is no medically approved drop-in power supply that simply resolves all of your team’s challenges. Risk assessment and review of your applied parts, and the usage conditions, are always required. This leads the design team to approaches that fulfill the medical requirements of the application and meet the other design parameters.
However, what helps is working with a power supply vendor with experience in this application domain. They should have a QMS meeting ISO 13485 covering both their design and manufacturing processes. But this is the bare minimum, so it is worth exploring what else they undertake to ensure high product safety and quality levels. Furthermore, as part of your risk assessment, they may also be able to deliver valuable additional data. It is worth exploring the availability of reports covering items such as the outcome of insulation breakdown, flammability, the impact of fan failure, mechanical shock, and inverted operation.